不太常见的非结核分枝杆菌肺部疾病的共识管理建议(可用浏览器右键翻译直接查看,比PDF方便)

美国胸科学会、欧洲呼吸学会、欧洲临床微生物学和传染病学会、美国传染病学会2020年非结核分枝杆菌肺病(NTM-PD)治疗临床实践指南;英国胸科学会的 2017 年管理指南涵盖了由鸟分枝杆菌复合体、堪萨斯分枝杆菌、异种分枝杆菌和脓肿分枝杆菌引起的成人肺部疾病。为了提供基于证据的建议,用于治疗没有囊性纤维化或 HIV 感染的成人患者中不太常见的非结核分枝杆菌 (NTM),我们的专家小组进行了系统的文献检索,为另外七种微生物引起的肺部疾病提供管理指导:龟分枝杆菌、偶发分枝杆菌、热分枝杆菌、戈登分枝杆菌、马尔默分枝杆菌、模拟分枝杆菌和舒尔盖分枝杆菌。治疗建议是通过结构化的共识过程制定的。以英文发表的关于其他 NTM 物种引起的肺部疾病治疗建议的科学文献的证据质量非常低,除 M malmoense 外,并且基于对病例报告和病例系列的评估。对于 M malmoense,两项随机对照试验和三项回顾性队列研究的结果为治疗建议提供了更好的证据基础
Summary
The 2020 clinical practice guideline for the treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) by the American Thoracic Society, European Respiratory Society, European Society of Clinical Microbiology and Infectious Diseases, and Infectious Diseases Society of America; and the 2017 management guideline by the British Thoracic Society covered pulmonary diseases in adults caused by Mycobacterium avium complex, Mycobacterium kansasii, Mycobacterium xenopi, and Mycobacterium abscessus. In order to provide evidence-based recommendations for the treatment of less common non-tuberculous mycobacterial (NTM) species in adult patients without cystic fibrosis or HIV infection, our expert panel group performed systematic literature searches to provide management guidance for pulmonary diseases caused by seven additional organisms: Mycobacterium chelonae, Mycobacterium fortuitum, Mycobacterium genavense, Mycobacterium gordonae, Mycobacterium malmoense, Mycobacterium simiae, and Mycobacterium szulgai. Treatment recommendations were developed by a structured consensus process. The evidence from the scientific literature published in English for treatment recommendations for pulmonary diseases caused by other NTM species was of very low quality, with the exception of M malmoense, and based on the evaluation of case reports and case series. For M malmoense, results from two randomised controlled trials and three retrospective cohort studies provided a better evidence base for treatment recommendations, although the evidence was still of low quality.
Introduction
The 2020 updated management guideline for patients with non-tuberculous mycobacterial pulmonary diseases (NTM-PD) focuses on population, intervention, comparison, and outcome question-guided management recommendations for pulmonary disease in adults caused by Mycobacterium avium complex, Mycobacterium kansasii, Mycobacterium xenopi, and Mycobacterium abscessus.1, 2 However, management options for NTM-PD caused by other clinically relevant non-tuberculous mycobacteria (NTM) covered in the previous management guideline are also needed for the care of affected patients.3 At present, treatment recommendations for patients with other NTM-PDs are primarily based on expert opinions and often variable. Health-care providers of patients with NTM-PDs should consult with a clinical microbiologist who has expertise in identification and antimycobacterial drug susceptibility testing, and a clinician with expertise in managing NTM disease. However, evidence-based management decisions are needed for patients affected by NTM not covered in the 2017 guideline by the British Thoracic Society (BTS) or the 2020 American Thoracic Society (ATS), European Respiratory Society (ERS), European Society of Microbiology and Clinical Infectious Diseases (ESCMID), and Infectious Diseases Society of America (IDSA) guideline.4
The panel of members of the 2020 ATS, ERS, ESCMID, and IDSA guideline committee did systematic reviews of the literature, independently of the societies involved in the original task force, to provide management guidance for pulmonary diseases caused by seven additional organisms: Mycobacterium chelonae, Mycobacterium fortuitum, Mycobacterium genavense, Mycobacterium gordonae, Mycobacterium malmoense, Mycobacterium simiae, and Mycobacterium szulgai.
The following consensus guidance includes recommendations for antibiotics guided by in-vitro susceptibility results. With the exception of M chelonae and M fortuitum, there are no validated break points defining susceptibility and resistance for any of the antibiotics used for the organisms considered here.5
Methods
A search (see appendix pp 4–7) was adapted for execution on the Ovid MEDLINE platform and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Embase, and the Cochrane Central Register of Controlled Trials. Searches were limited to human studies or studies indexed with neither human nor animal and those published in English. A final update was executed on Jan 6, 2020. Teams of two or three independent experts evaluated the search results for eligibility; they supplemented the electronic search by contacting experts and handsearching journals, conference proceedings, reference lists, and regulatory agency websites for relevant articles.
In addition to the systematic reviews, the members of the panel ascertained agreement on management options in a six-step consensus process as previously published.6, 7, 8 The results of the panel experts' votes, either in favour or against the treatment approach, is presented in the appendix (appendix p 1).
General recommendations
The expert panel recommends that health-care providers consult with a clinical microbiologist who has expertise in identification and antimycobacterial drug susceptibility testing. Patients should be referred to a clinician with expertise in managing NTM-PD before treatment against any of the mycobacterial pathogens described in this consensus document is initiated. The clinical and microbiological criteria for the diagnosis of NTM-PD are displayed in panel.
Panel
Clinical and microbiological criteria for diagnosis of non-tuberculous mycobacteria
Clinical
Pulmonary or systemic symptoms; and nodular or cavitary opacities on chest radiograph or a high-resolution computed tomography scan that shows bronchiectasis with multiple small nodules and appropriate exclusion of other diagnoses.
Microbiological*•
Positive culture results for a non-tuberculous mycobacteria (NTM) from at least two separate expectorated sputum samples. If the results are nondiagnostic, consider repeat sputum acid-fast bacilli smears and cultures.
or•
Positive culture results for a NTM from at least one bronchial wash or lavage.
or•
Transbronchial or other lung biopsy with mycobacterial histologic features (granulomatous inflammation or acid-fast bacilli) and positive culture for NTM or biopsy showing mycobacterial histologic features (granulomatous inflammation or acid-fast bacilli) and one or more expectorated sputum or bronchial washings that are culture positive for NTM.
Expert consultation should be obtained when NTM are recovered that are either infrequently encountered or that usually represent environmental contamination. Patients who are suspected of having NTM pulmonary disease but do not meet the diagnostic criteria should be followed up until the diagnosis is firmly established or excluded. Making the diagnosis of NTM pulmonary disease does not per se, necessitate the institution of therapy, which is a decision based on the potential risks and benefits of therapy for individual patients.
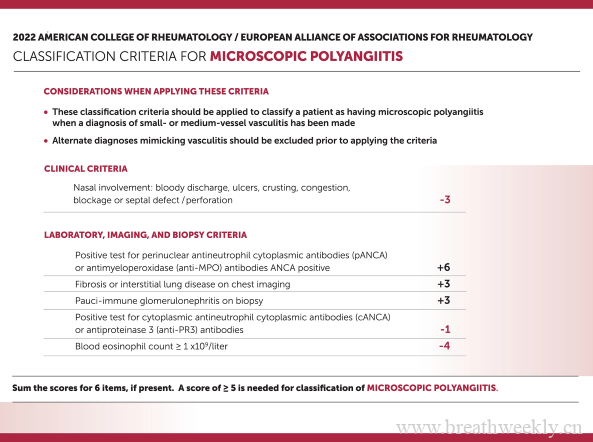
The choice of treatment regimen should be guided by the results of the antimycobacterial drug susceptibility testing, although for some species causing NTM-PD—ie, M genavense and M simiae—the correlation of antimycobacterial drug susceptibility testing and clinical outcome can be poor (figure). For orientation, the minimum inhibitory concentrations (MIC) of antibiotics against select NTM species required to inhibit the growth of 50% and 90% of organisms (MIC50 and MIC90) are provided in the appendix (appendix p 2). The major contributions of the mycobacteriology laboratory are to correctly identify the NTM species, perform antimycobacterial drug susceptibility testing, and detect acquired and inducible resistance. The dosing of drugs for the treatment of NTM-PDs mentioned in this document follows recent ATS, ERS, ESCMID, and IDSA recommendations (table 1).1, 2 Treatment considerations are summarised in Table 2, Table 3 and the quality of the evidence and strength of the recommendations are displayed in the appendix (appendix p 3)
![图片[2]-不太常见的非结核分枝杆菌肺部疾病的共识管理建议 | 每周呼吸-每周呼吸](https://webvpn.jlu.edu.cn/https/77726476706e69737468656265737421f1e552d2223c7b1d7d0c87e29b5a2e/content/image/1-s2.0-S1473309921005867-gr1.jpg)
Table 1. Dosing guidelines for drugs used in the management of NTM-PD
| Empty Cell | Daily dose | Three times a week dose | Hepatic impairment dose | Renal impairment dose |
|---|---|---|---|---|
| Oral | ||||
| Azithromycin | 250–500 mg once a day | 500 mg once a day | NA | NA |
| Ciprofloxacin | 500–750 mg twice a day | NA | NA | 250–500 mg dosed at intervals according to CrCl |
| Clarithromycin | 500 mg twice a day | 500 mg once a day | NA | Reduce dose by 50% if CrCl less than 30 mL/min |
| Clofazimine* | 100–200 mg once a day | NA | Caution in severe hepatic impairment | NA |
| Doxycycline | 100 mg once a day or twice a day | NA | NA | NA |
| Ethambutol | 15 mg/kg once a day | 25 mg/kg once a day | NA | Increase dosing interval (eg, 15–25 mg/kg, three times a week) |
| Levofloxacin | 750–1000 mg once a day | NA | NA | Increase dosing interval (eg, 10–15 mg/kg, three times a week) |
| Linezolid | 600 mg once a day or twice a day† | NA | NA | NA |
| Moxifloxacin | 400 mg once a day | NA | NA | NA |
| Rifabutin | 150–300 mg once a day (150 mg once a day with clarithromycin) | 300 mg once a day | Caution | Reduce dose by 50% if CrCl less than 30 mL/min |
| Rifampicin | 10 mg/kg (450 mg or 600 mg) once a day | 600 mg once a day | Caution | NA |
| Co-trimoxazole | 800 mg trimethoprim and 160 mg sulfamethoxazole twice a day | NA | Caution | Reduce dose by 50% if CrCl 15–30 mL/min |
| Parenteral | ||||
| Amikacin‡ (IV) | 15–20 mg/kg once a day§, adjusted according to drug level monitoring¶ | 15–25 mg/kg once a day§, adjusted according to drug level monitoring¶ | NA | Reduce dose or increase dosing interval (eg, 15 mg/kg, twice a week or three times a week) |
| Cefoxitin (IV) | 2–4 g twice a day or three times a day (maximum daily dose is 12 g/day) | NA | NA | Reduce dose or increase dosing interval |
| Imipenem-cilastatin (IV) | 1g each, twice a day or three times a day | NA | NA | Reduce dose or increase dosing interval |
| Tobramycin‡ (IV) | 4.5–7 mg/kg once a day | 5–7 mg/kg once a day | NA | Reduce dose or increase dosing interval (eg, 5–7 mg/kg, twice a week or three times a week); inhalative route of administration not studied |
NA=not applicable. NTM-PD= non-tuberculous mycobacterial pulmonary disease. CrCl= creatinine clearance. IV=intravenously. This table should be considered as a general guide for dosing in clinical practice, and it does not replace specific local, national, or regional dosing guidelines.*
Clofazimine availability varies by country. In the USA, an investigational new drug application is required.†
Most experts recommend once a day dosing of linezolid because of the high rate of drug-related adverse reactions associated with twice a day dosing.‡
Caution must be exercised in the prolonged use of aminoglycosides because of the risk of otovestibular toxicity and nephrotoxicity.§
The use of the described regimens for 15 weeks was associated with permanent ototoxicity in approximately a-third of patients, and the risk was associated with age and cumulative dose. Given the high rates of ototoxicity, risks and benefits should be carefully considered with the aims of therapy. Clinicians should consider lower dose ranges and probably rely on intermittent dosing when more prolonged therapy is employed.¶
Drug level monitoring: trough less than 5 mg/L, peak with daily dosing 35–45 μg/mL, peak with intermittent dosing 65–80 μg/mL.
Table 2. Treatment regimens for rapidly growing NTM-PD
| Empty Cell | Number of drugs | Drugs | Duration of therapy | Comments |
|---|---|---|---|---|
| M chelonae | Initial phase (≥3); continuation phase (≥2) | Azithromycin (once a day) or clarithromycin (twice a day); tobramycin IV (once a day or three times a week); imipenem-cilastatin (two to three times a day); moxifloxacin (once a day) or levofloxacin (once a day), or ciprofloxacin (twice a day); linezolid (once a day); clofazamine (once a day) | 12 months beyond culture conversion | Very low level of evidence; drugs should be selected according to DST results when available; caution patients about aminoglycoside ototoxicity and nephrotoxicity; for mild to moderate disease an oral two-drug regimen could suffice, provided that DST has proven two such drugs to be active |
| M fortuitum | Initial phase (≥3); continuation phase (≥2) | Moxifloxacin (once a day) or levofloxacin (once a day), or ciprofloxacin (twice a day); amikacin IV (once a day or three times a week); imipenem-cilastatin (two to three times a day); cefoxitin (two to three times a day); linezolid (once a day); co-trimoxazole (twice a day); (clofazimine [once a day]); (doxycycline [twice a day])* | 12 months beyond culture conversion | Very low level of evidence; drugs should be selected according to DST results when available; the detection and management of underlying oesophageal disorders or aspiration is critical; fluoroquinolones are probably the most effective; there is natural resistance to macrolides; caution patients about ototoxicity and nephrotoxicity of aminoglycosides; for mild to moderate disease an oral two-drug regimen could suffice provided that DST has proven two such drugs to be active |
NTM-PD=non-tuberculous mycobacterial pulmonary disease. IV=intravenously. DST=drug susceptibility testing.*
Drugs in brackets are non-preferred options.
Table 3. Treatment regimens for slowly growing NTM-PD
| Empty Cell | Number of drugs | Drugs* | Duration of therapy | Comments |
|---|---|---|---|---|
| M genavense | ≥3 | Azithromycin (once a day) or (clarithromycin [twice a day]); rifampicin (once a day); ethambutol (once a day); (moxifloxacin [once a day]); (clofazimine [once a day]); (amikacin IV [once a day]) | 12 months beyond culture conversion | Very low level of evidence; drugs should be selected according to DST results when available; moxifloxacin or amikacin may be used in cases of intolerance or drug resistance to macrolides, rifamycins, or ethambutol |
| M malmoense | ≥3 | Azithromycin (once a day) or (clarithromycin [twice a day]); rifampicin (once a day); ethambutol (once a day); (amikacin IV [once a day or three times a week]); (moxifloxacin [once a day]); (clofazimine [once a day]) | 12 months beyond culture conversion | Low level of evidence; drugs should be selected according to DST results, when available; moxifloxacin or clofazimine can be used in case of intolerance or drug resistance to macrolides, rifamycins, or ethambutol; amikacin should be added for cavitary or severe disease; caution patients about aminoglycoside ototoxicity and nephrotoxicity |
| M szulgai | ≥3 | Azithromycin (once a day) or (clarithromycin [twice a day]); rifampicin (once a day); ethambutol (once a day); (moxifloxacin [once a day]); (clofazimine [once a day]); (amikacin IV [once a day or three times a week]) | 12 months or 12 months beyond culture conversion if treatment with a macrolide, a rifamycin, or ethambutol cannot be used. | Very low level of evidence; drugs should be selected according to DST results, when available; fluoroquinolones (moxifloxacin or levofloxacin), clofazimine, or aminoglycosides (streptomycin or amikacin) can be used in case of intolerance or drug resistance to macrolides, rifamycins, or ethambutol; caution patients about ototoxicity and nephrotoxicity of aminoglycosides |
| M simiae | ≥3 | Azithromycin or (clarithromycin); moxifloxacin (once a day); co-trimoxazole (twice a day); clofazimine (once a day); (amikacin IV [once a day or three times a week]) | 12 months beyond culture conversion | Very low level of evidence; drugs should be selected according to DST results, when available; amikacin should be added for cavitary or severe disease; possible combinations include azithromycin, moxifloxacin, and co-trimoxazole; azithromycin, clofazimine, and amikacin; or azithromycin and moxifloxacin in combination with one or two additional drugs based on DST results with clofazimine and amikacin being the most suitable options |
| M gordonae† | NA | NA | NA | NA |
IV=intravenously. DST=drug susceptibility testing. NA=not applicable.*
Drugs in brackets are non-preferred options.†
In general, isolation of M gordonae from respiratory specimens represents transient colonisation or laboratory contamination; treatment should not be offered without overwhelming evidence that this organism is causing invasive pulmonary disease. If treatment is necessary, DST can be performed; although no standard treatment can be recommended, a combination of a macrolide, rifampicin, and ethambutol has been described as successful.
To monitor treatment (supplement table 2, appendix p 3), sputum samples should be collected for culture every 1–2 months after initiation of therapy until there is sputum conversion to culture negative (at least two consecutive negative mycobacterial sputum cultures, collected at least 4 weeks apart during antibiotic treatment); the sampling date of the first negative culture is the date of sputum conversion to culture negative.6 Thereafter, sputum should be collected every 2–3 months until therapy is completed, defined as 12 months of negative mycobacterial sputum cultures (while on therapy) from the date of the first negative culture.
Rapidly growing NTM
M chelonae
M chelonae is an unusual cause of NTM-PD;3, 9, 10 therefore, clinicians should carefully assess for other causes of the patients symptoms and verify fulfillment of disease criteria before embarking on a course of treatment. Isolates are usually susceptible to tobramycin, macrolides (eg, clarithromycin and azithromycin), clofazimine, linezolid, and sometimes to fluoroquinolones and imipenem.3, 11, 12, 13, 14, 15 Tobramycin is more active in vitro than amikacin and M chelonae is generally resistant to cefoxitin.16 M chelonae does not contain an erythromycin resistance methylase (erm) gene,17 so the macrolides are considered fully active, unlike M fortuitum and some M abscessus subspecies, which have functional erm genes. Antimycobacterial drug susceptibility testing should be done, as well as detection of acquired resistance to macrolides, which was described in this species, although mostly in non-pulmonary infections.18
A systematic literature review by two independent experts identified 18 case reports and case series describing 57 patients with M chelonae pulmonary disease in the scientific literature published in English, but no evidence-based management recommendations for M chelonae pulmonary disease based on clinical trial data.9, 14, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 In most patients, risk factors for M chelonae pulmonary disease were not stated or could not be identified. Approximately 15% of patients who have been reported with M chelonae pulmonary disease were post-organ transplant.19, 21, 23 By contrast with M fortuitum pulmonary disease, gastrointestinal motility disorders (eg, achalasia) did not appear to be a prominent risk factor for M chelonae pulmonary disease. The clinical spectrum of M chelonae pulmonary disease includes bronchiolitis, fibronodular or patchy consolidations, cavitary disease, and rarely empyema.
Treatment for M chelonae pulmonary disease initially includes at least two drugs for mild to moderate disease or three drugs in more severe disease that the organism shows in-vitro susceptibility to. Treatment often includes one or two intravenous drugs to be continued for an initial period, usually for 4–16 weeks, to achieve control of disease; tobramycin is the preferred aminoglycoside. At least two oral drugs, one of which should be a macrolide that has demonstrated in-vitro susceptibility, are administered for a total duration of therapy of at least 12 months after conversion to culture negative, to complete the treatment regimen.
Favoured antibiotics include azithromycin (250–500 mg) once a day or clarithromycin (500 mg) twice a day, tobramycin (4·5–7 mg/kg) intravenously once a day or tobramycin (5–7 mg/kg) intravenously three times a week, imipenem-cilastatin (1 g each) intravenously two or three times a day or a fluoroquinolone—eg, moxifloxacin (400 mg) once a day—clofazimine (100–200 mg) once a day, or linezolid (600 mg) once a day, based on in-vitro antimycobacterial drug susceptibility test results. Caution must be exercised in the prolonged use of aminoglycosides because of the risk of otovestibular toxicity and nephrotoxicity. Approximately 20% of patients with M chelonae pulmonary disease received adjunctive partial pulmonary surgical resection;20, 25 these patients generally had a successful treatment outcome, thus surgical resection should be considered when it is an option. Based on the available literature, it was not possible to state an association between the type of drug therapy against M chelonae pulmonary disease and successful treatment outcome.
M fortuitum
Although M fortuitum is a relatively common NTM species isolated from respiratory specimens, it is a relatively uncommon cause of NTM-PD.3, 9, 35 Clinicians should maintain a high-diagnostic threshold for identifying M fortuitum pulmonary disease, and they should carefully assess for other causes of the patients symptoms and radiological abnormalities. M fortuitum isolates are usually susceptible to multiple antibiotics including fluoroquinolones, doxycycline, minocycline, and sulfonamides.3 In-vitro data suggest susceptibility to clofazimine (appendix p 2). In addition, isolates are typically susceptible to amikacin and imipenem. M fortuitum usually contains an erm (39) gene which confers inducible resistance; therefore, macrolides should not be considered active.36 Antimycobacterial drug susceptibility testing should be done, as well as detection of acquired resistance to fluoroquinolones, which was described in this species, although mostly in non-pulmonary infections.37
A systematic literature review by two independent experts identified 45 case reports and case series describing 150 patients with M fortuitum pulmonary disease in the scientific literature published in English, but no evidence-based management recommendations based on clinical trial data for M fortuitum pulmonary disease.11, 19, 21, 24, 26, 28, 29, 31, 32, 34, 35, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71
M fortuitum pulmonary disease occurs predominantly in patients with underlying conditions: oesophageal and gastrointestinal motility problems (eg, achalasia),40, 41, 45 structural lung changes such as post-tuberculosis changes35, 38, 54 severe chronic obstructive pulmonary disease (COPD),58, 69 and cancer.24, 58 Conversion to sputum culture negative for M fortuitum without antibiotic treatment is not uncommon when the underlying cause (eg, achalasia) has been addressed.9 The underlying cause must be addressed if possible; with its correction or improvement, antibiotic treatment might not be required, or a less intensive regimen might suffice. However, M fortuitum pulmonary disease is associated with a poor prognosis and about a-third of the affected patients reported in the literature do not survive despite antibiotic therapy, probably reflecting the severity of the underlying disease.28, 45, 58, 68, 71
Patients who received fluoroquinolones as part of their treatment regimen seemed to have a favourable treatment outcome, but we did not find any publications that met the criteria of a clinical trial for evidence-based management recommendations for M fortuitum pulmonary disease.38, 42, 53, 54, 56, 58, 63, 67
Clinicians should be aware that there are other mycobacterial species related to M fortuitum (Mycobacterim peregrinum, Mycobacterim septicum, Mycobacterim porcinum, and Mycobacterim conceptionense) included in the so-called M fortuitum complex, which might present different antibiotic susceptibilities, including macrolide susceptibility.72
Treatment for M fortuitum pulmonary disease should include at least two drugs for mild-to-moderate disease or three drugs in more severe disease that the organism shows in-vitro susceptibility to. Treatment often includes one or two intravenous drugs to be continued for an initial period, usually for 4–16 weeks, to achieve control of disease; amikacin is the preferred aminoglycoside. Frequently used doses include amikacin (15–20 mg/kg) intravenously once a day or amikacin (15–25 mg/kg) three times a week in severe disease manifestations (lower in older patients and in prolonged treatment). Caution must be exercised in the prolonged use of aminoglycosides because of the risk of otovestibular toxicity and nephrotoxicity. Other favoured intravenous drugs are imipenem-cilastatin (1 g each) intravenously two or three times a day or cefoxitin (3–4 g) intravenously twice a day. At least two oral drugs that have demonstrated in-vitro susceptibility should be continued until at least 12 months after conversion to culture negative to complete the treatment regimen. Favoured oral antibiotics include fluoroquinolones—eg, moxifloxacin (400 mg) once a day or levofloxacin (750–1000 mg) once a day—co-trimoxazole (trimethoprim 800 mg and 160 mg sulfamethoxazole) twice a day, linezolid (600 mg) once a day, clofazimine (100–200 mg) once a day, or doxycycline (100–200 mg) once a day. A summary of rapidly growing NTM treatment recommendations is shown in table 2.
Slowly growing NTM
M genavense
M genavense is a rare cause of NTM-PD.73, 74, 75 The organism is fastidious and does not grow on solid media, although limited growth can be observed in supplemented broth cultures; molecular detection is most reliable, thus antimycobacterial drug susceptibility testing in M genavense is challenging.76 A systematic literature review by two independent experts identified five case reports and case series describing six patients with M genavense pulmonary disease in the scientific literature published in English, but no clinical trial data to inform evidence-based management recommendations for M genavense pulmonary disease.73, 77, 78, 79 M genavense was first described in the setting of severe immunosuppression with disseminated disease.3, 74, 75, 80
Available data suggests that most isolates are susceptible in vitro to fluoroquinolones, clofazimine, and amikacin, although susceptibility testing has not been standardised for M genavense (appendix p 2).80, 81 Case series have described treatment success with macrolide containing multi-drug regimens that typically include ethambutol plus one or two other drugs such as a rifamycin, amikacin, or a fluoroquinolone.74, 75, 82, 83, 84
Treatment for M genavense pulmonary disease includes at least three drugs that the organism shows in-vitro susceptibility to. The panel suggests the following treatment regimen: azithromycin (250–500 mg) once a day, rifampicin (600 mg) once a day, and ethambutol (15 mg/kg) once a day. In case of intolerance or drug resistance to macrolides, rifamycins, or ethambutol, the following treatment regime can be used instead: moxifloxacin (400 mg) once a day, amikacin (15–20 mg/kg) intravenously once a day or amikacin (15–25 mg/kg) three times a week (lower in older patients and prolonged use), or clofazimine (100–200 mg) once a day. Treatment should be continued until at least 12 months post-conversion to culture negative to complete the treatment regimen.
M gordonae
M gordonae is known as a non-pathogenic species but is one of the most common NTM isolated from respiratory samples; probably because it is prevalent in the environment, especially the water supply network.85, 86, 87, 88, 89 It is also easily isolated in mycobacteriology laboratories (naturally orange colonies growing on all media after 2 weeks incubation). Limited data suggest that M gordonae is variably susceptible in vitro to several agents: clarithromycin, ciprofloxacin, linezolid, and amikacin.90, 91, 92 The correlation between these in-vitro results and patient outcomes is unknown.
A systematic literature review by two independent experts identified nine case reports and case series describing 13 non-immunosuppressed adults with M gordonae pulmonary disease in whom treatment and outcomes were adequately described, but no evidence-based management recommendations for M gordonae pulmonary disease based on clinical trial data.69, 93, 94, 95, 96, 97, 98, 99, 100
Treatment for M gordonae pulmonary disease before 1990 often included isoniazid, rifampicin, and ethambutol. Treatment in newer studies more frequently included clarithromycin, rifampicin, and ethambutol. Information regarding cure was provided for 12 cases and was definite or likely in 11 (92%) patients overall and 11 patients who did not experience respiratory failure.69, 93, 94, 95, 96, 97, 98, 99, 100 Surgical resection, in addition to antibiotic therapy, was described in two patients, both of whom were cured.97, 100
When M gordonae is cultured in respiratory samples, other causes of lung disease should be carefully considered. Multiple sputum samples should be collected over several weeks or months to identify the presence of any other NTM known to be more pathogenic. Clinicians should maintain a high-diagnostic threshold for identifying M gordonae pulmonary disease. In addition to the clinical and radiological criteria for M gordonae pulmonary disease (as M gordonae is almost always non-pathogenic) described in the international NTM guidelines,97 more than two positive sputum cultures and at least one detection of acid-fast bacilli in a respiratory specimen with documented M gordonae cultures should be present.
Even when current diagnostic criteria are met, treatment is seldom necessary. In the rare situation when treatment is necessary, antimycobacterial drug susceptibility testing can be done as for other NTM; although no standard treatment can be recommended, a combination of a macrolide, rifampicin, and ethambutol has been described as successful in some cases.
M malmoense
M malmoense was first described in four patients from Malmö and Lund, Sweden,101 in 1977 and is one of the most common species causing NTM-PD in regions of northern Europe. Multiple studies using different laboratory methods have described the susceptibility of M malmoense to rifampicin, rifabutin, clarithromycin, and clofazimine, with variable resistance to amikacin, ethambutol, and fluoroquinolones, and resistance to isoniazid.102, 103, 104, 105, 106
M malmoense most commonly causes pulmonary disease but can also cause cervical lymphadenitis, tenosynovitis, skin infections, and rarely disseminated disease in the severely immunocompromised host. It is seldom isolated from the environment but it can be found in natural water and soil in some geographical areas.107, 108, 109 The clinical presentation of M malmoense pulmonary disease frequently mimics tuberculosis, with cavities and airspace disease being the most common radiological findings.110, 111 The majority of affected patients are male, approximately half have underlying COPD or a history of pulmonary tuberculosis. When M malmoense is isolated from respiratory samples, it is usually of clinical significance, although there is some geographical variability suggesting that pathogenicity might vary by region.102, 112
A systematic literature review by two independent experts found five publications (two randomised controlled trials104, 105 and three retrospective cohort studies102, 113, 114) that met criteria for evidence-based management recommendations for M malmoense pulmonary disease. In addition, two systematic reviews were identified that addressed treatment outcomes or treatment recommendations for M malmoense pulmonary disease.4, 48
An early retrospective review113 from Cardiff, UK, described the clinical outcomes of 37 patients with M malmoense pulmonary disease who were treated with a variety of non-macrolide-based treatment regimens. Those who received three drugs (isoniazid, rifampicin, and ethambutol) for 18–24 months tended to do better than those who received two drugs, or those who were treated for less than 18 months.113 Among patients treated with a BTS recommended regimen (rifampicin plus different combinations of ethambutol, isoniazid, clarithromycin, or ciprofloxacin) treatment success was achieved in 75% of patients. In a series of 14 consecutive patients with fibrocavitary M malmoense pulmonary disease in Edinburgh, UK, investigators reported 100% conversion to culture negative and symptom reduction after 24 months of combination rifampicin (450–600 mg) once a day, ethambutol (15 mg/kg) once a day, and clarithromycin (500 mg) twice a day.114 In this study initial M malmoense isolates from all 14 patients were judged to be susceptible to rifampicin and resistant to isoniazid, with 23% resistant to ethambutol, 8% resistant to clarithromycin, and 46% resistant to ciprofloxacin. A retrospective review of 30 patients with M malmoense pulmonary disease from the Netherlands found that multi-drug treatment regimens varied considerably, although most received a macrolide in addition to ethambutol and rifampicin.102 The mean duration of therapy was 12 months, which is shorter than the duration in the BTS trials. 21 (70%) patients had a good clinical response (defined as symptomatic improvement and conversion to culture negative) to treatment, five (17%) patients had failure or relapse, and four (13%) patients died.
The BTS performed a randomised controlled trial involving 106 patients with M malmoense pulmonary disease; patients were treated for 2 years with either rifampicin and ethambutol or rifampicin, ethambutol, and isoniazid.104, 105 Of 106 patients, 10% of patients had a poor clinical outcome (death due to NTM, treatment failure, or relapse); there was no significant difference between groups. Only 20 (38%) of 52 patients who received rifampicin and ethambutol, and 24 (44%) of 54 of patients who additionally received isoniazid were alive and free of relapse, and therefore considered cured after 5 years. A second BTS randomised controlled trial allocated 167 patients to 2 years of treatment with rifampicin, ethambutol, and clarithromycin; or rifampicin, ethambutol, and ciprofloxacin. 115 Although there was no significant difference in the poor outcome rate between groups (overall, 7%), more patients receiving the rifampicin, ethambutol, and clarithromycin regimen completed treatment and were alive and cured at 5 years (38% vs 20%); however, there were more side effects in this group. In addition, the number of patients alive and cured at 5 years in the clarithromycin group was the same as that seen with rifampicin and ethambutol in the previous trial. Compared with patients with M avium complex and M xenopi pulmonary disease, who were also included in the trial, patients with M malmoense were significantly less likely to have a poor outcome.
Two systematic reviews were identified.4, 48 One systematic review reported that three studies105, 114, 115 investigated treatment outcomes in patients with M malmoense pulmonary disease, comprising a total of 287 patients.48 The weighted average proportion of treatment success (the proportion of patients in whom sputum conversion to culture negative was achieved at the end of therapy minus the proportion of relapses reported at the end of follow-up) was 54·4% (95% CI 34·7–73·4); however, the authors noted that the comparability between the studies and the different treatment regimens was impeded by the high all-cause mortality of 34–49·1%.
The BTS NTM guideline reviewed two randomised controlled trials104, 115 and four case series.102, 103, 113, 114 For non-cavitary disease the recommended treatment was with a macrolide, rifampicin, and ethambutol. The addition of parenteral or inhaled amikacin was recommended in cavitary disease, although there was no evidence presented that supported the use of amikacin.4 A macrolide-containing three-drug regimen offers the best option for treatment success based on these studies, although treatment outcomes remain suboptimal and the evidence base for treatment recommendations is low.
Treatment for M malmoense pulmonary disease generally includes at least three drugs: azithromycin (250–500 mg) once a day or clarithromycin (500 mg) twice a day, rifampicin (600 mg) once a day, and ethambutol (15 mg/kg) once a day. Additional drugs may include moxifloxacin (400 mg) once a day or clofazimine (100–200 mg) once a day. Based on the utility of amikacin for most NTM species (except M chelonae), favourable in-vitro drug susceptibility profiles, and expert opinion, the addition of parenteral amikacin can be considered in severe cases, such as cavitary disease. The optimal duration of therapy is unknown, but consistent with other NTM pulmonary pathogens the expert panel recommends a total duration of therapy of at least 12 months post-conversion to culture negative to complete the treatment regimen.
M simiae
M simiae is a rare human pathogen causing disseminated or pulmonary disease; only 4–21% of patients with respiratory M simiae isolates fulfil the ATS diagnostic criteria.116, 117, 118 The rates of isolation vary geographically119 with higher rates being reported in Israel120, 121, Lebanon122, Iran123 and India.124 Respiratory isolates of M simiae are most often contaminants, thus clinicians should maintain a high threshold to diagnose M simiae pulmonary disease and carefully assess for other causes of a patient's symptoms or radiological abnormalities. M simiae is considered among the hardest to treat NTM owing to its natural drug resistance; most antimycobacterial drug susceptibility testing reports of M simiae describe resistance to most tested drugs.116, 118, 125 In vitro, the M simiae strain ATCC 25275 was resistant to most of the 19 drugs tested, except macrolides, clofazimine, and sulfamethoxazole (quinolones and aminoglycosides were not tested).126 Furthermore, there is in-vitro synergy between clofazimine and amikacin for M simiae isolates.11 In addition, variable proportions of clinical strains can be susceptible to moxifloxacin (and to ciprofloxacin, to a lesser extent), aminoglycosides, and cycloserine.125, 127
A systematic literature review by two independent experts identified 11 case reports and case series describing 197 patients with M simiae pulmonary disease in the scientific literature published in English, but no evidence-based management recommendations for M simiae pulmonary disease based on clinical trial data.116, 117, 121, 122, 128, 129, 130, 131, 132, 133, 134 The largest study of 102 patients was from Israel; no treatment failures or relapses were reported during a mean follow-up of 24 months, with patients receiving clarithromycin, ethambutol, and rifampicin treatment for at least 12 months.121 Although the 1997 ATS criteria were reportedly used to select patients, 38 (37%) of 102 patients were asymptomatic and had an unremarkable chest radiograph; therefore, not fulfilling these criteria. The expert panel concluded that data from this study could not be considered reliable enough to form a basis for treatment recommendations.121
The treatment regimen for M simiae pulmonary disease includes at least three drugs: azithromycin (250–500 mg) once a day or clarithromycin (500 mg) twice a day, moxifloxacin (400 mg) once a day, co-trimoxazole (trimethoprim 800 mg and sulfamethoxazole 160 mg) twice a day, amikacin (15–20 mg/kg) intravenously once a day or amikacin (15–25 mg/kg) three times a week (lower in older patients and with prolonged use), or clofazimine (100–200 mg) once a day. Depending on antimycobacterial drug susceptibiltity testing, accepted treatment regimes could be a clofazimine, amikacin, and azithromycin combination or a azithromycin, moxifloxacin, and co-trimoxazole combination,3, 16 or a azithromycin and moxifloxacin combination with one or two additional drugs based on antimycobacterial drug susceptibility testing results; clofazimine and aminoglycosides would be the most appropriate options.125 Surgical resection of affected lobes should be evaluated as an adjunctive treatment option.16 Optimal treatment duration is unknown; experts consider treating for at least 12 months after conversion to negative sputum culture, possibly introducing a step-down approach to consolidation therapy (eg, stop intravenous amikacin) once clinical, microbiological, and radiological improvement has been documented.
M szulgai
M szulgai is an unusual cause of pulmonary disease and accounts for less than 1% of human respiratory isolates of NTM; however, the isolation of M szulgai appears to be clinically relevant in most cases.135, 136, 137 Isolates are usually susceptible in vitro to clarithromycin and rifampicin. The largest case series reporting on antimycobacterial susceptibility testing of M szulgai included 23 strains of M szulgai.106 Drug resistance was observed in all 23 (100%) strains to isoniazid, in 9% of strains to ethambutol, in 26% of strains to ciprofloxacin, 13% of strains to amikacin, and in 4% of strains to rifampicin. All 23 strains of M szulgai were susceptible to clarithromycin. In-vitro data suggest susceptibility to clofazimine (appendix p 2). Later generation fluoroquinolones were not tested.
A systematic literature review conducted by two independent experts identified 25 retrospective case reports and case series, including a total of 44 patients with M szulgai pulmonary disease described in the scientific literature published in English136, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149 but no evidence-based management recommendations for M szulgai pulmonary disease based on clinical trial data. Most patients were treated with a combination of rifampicin, ethambutol, and clarithromycin or azithromycin. Other treatments included a mixture of rifampicin-based regimens; usually a combination of rifampicin with two or more drugs: isoniazid, ethambutol, fluoroquinolones, ethionamide, and intravenous drugs. Treatment duration was variable; 12 months was most frequently used (range 5–18 months). The outcome was favourable in 85% of patients treated with rifampicin, clarithromycin, and azithromycin combination regimens; no relapses were observed among the five (11%) patients that post-treatment follow-up was available for. The cure rate was 81% among 21 patients treated with clarithromycin and azithromycin sparing regimens; unfavourable outcomes included one failure of treatment after 6 months and one relapse related to non-compliance. Four (9%) patients underwent surgery, but it was only performed to treat M szulgai pulmonary disease in two of these patients, and one of these two patients had a tissue culture sample that was negative for M szulgai.
Treatment for M szulgai pulmonary disease should include at least three drugs that the organism shows in-vitro susceptibility for. Based on the low level evidence available, a combination of rifampicin (600 mg) once a day, azithromycin (250–500 mg) once a day or clarithromycin (500 mg) twice a day, and ethambutol (15 mg/kg) once a day for 12 months is recommended. Clofazimine (100–200 mg) once a day can be used if there is intolerance or drug resistance to macrolides, rifamycins, or ethambutol. Intravenously administered drugs (eg, amikacin) are alternatives in case of intolerance or resistance to the recommended orally available drugs. There are no in-vitro susceptibility data or clinical experience with later-generation fluoroquinolones. In such cases, if a treatment with a macrolide, rifamycin, and ethambutol cannot be used, it is advised to prolong treatment to 12 months after conversion to culture negative. There is no evidence to recommend surgery as part of the treatment for M szulgai pulmonary disease.
A summary of slowly growing NTM treatment recommendations is shown in table 3.
Conclusion
We did a systematic review of the literature and consensus process to provide treatment recommendations for pulmonary diseases caused by seven additional NTM not covered in the recent ATS, ERS, ESCMID, and IDSA or BTS clinical practice guidelines.1, 2, 4
With the exception of M malmoense, where recommendations are based on two randomised controlled trials and three retrospective cohort studies, the consensus recommendations by the panel members for the other six NTM species are based on case reports and case series only, thus are graded to be very low level evidence. Higher level evidence from patient registries and clinical trials to determine the best treatments for patients affected by pulmonary diseases caused by the other six NTM species are needed to inform clinicians in the future about the best management options for their patients.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on February 3, 2022
Contributors
CL, CLD, and EC developed the idea and outline for the manuscript. SLK performed the electronic systematic literature searches. All co-authors contributed equally to the hand-search of the selected literature, the consensus process, drafting of the manuscript, and manuscript revision.
Declaration of interests
CLD reports grants and personal fees from Insmed, Paratek, and Spero; personal fees from AN2 and Matinas, and grants from BugWorks; outside the submitted work. DEB reports grants and personal fees from Insmed. CL is supported by the German Center for Infection Research (DZIF) and reports personal fees from Chiesi, Gilead, Janssen, Novartis, Oxfordimmunotec, and Insmed outside the submitted work. TKM reports grants and personal fees from Insmed; and personal fees from Astra Zeneca, RedHill Biopharma, Novartis, and Spero; outside the submitted work. KNO is supported by the intramural research program of the NHLBI, NIH and reports grants from Beyond Air outside the submitted work. KW reports grants and personal fees from Insmed; and personal fees from Paratek, Red Hill Biopharma, Horizon, and Spero; outside the submitted work. All other authors declare no competing interests.
Acknowledgments
CL is supported by the German Center for Infection Research (DZIF). KNO is supported by the intramural research program of The National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH).
References
CL Daley, JM Iaccarino, C Lange, et al.
Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline
Eur Respir J, 56 (2020), Article 20005352
CL Daley, JM Iaccarino, C Lange, et al.
Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline
Clin Infect Dis, 71 (2020), pp. 905-9133
DE Griffith, T Aksamit, BA Brown-Elliott, et al.
An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases
Am J Respir Crit Care Med, 175 (2007), pp. 367-4164
CS Haworth, J Banks, T Capstick, et al.
British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD)
Thorax, 72 (suppl 2) (2017), pp. ii1-i645
BA Brown-Elliott, GL Woods
Antimycobacterial susceptibility testing of nontuberculous mycobacteria
J Clin Microbiol, 57 (2019), pp. e00834-e009196
J van Ingen, T Aksamit, C Andrejak, et al.
Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement
Eur Respir J, 51 (2018), Article 18001707
C Lange, I Abubakar, JW Alffenaar, et al.
Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement
Eur Respir J, 44 (2014), pp. 23-638
J Domínguez, EC Boettger, D Cirillo, et al.
Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: a TBNET/RESIST-TB consensus statement
Int J Tuberc Lung Dis, 20 (2016), pp. 24-429
DE Griffith, WM Girard, RJ Wallace Jr
Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients
Am Rev Respir Dis, 147 (1993), pp. 1271-127810
J van Ingen, R de Zwaan, RP Dekhuijzen, MJ Boeree, D van Soolingen
Clinical relevance of Mycobacterium chelonae-abscessus group isolation in 95 patients
J Infect, 59 (2009), pp. 324-33111
J van Ingen, SE Totten, NK Helstrom, LB Heifets, MJ Boeree, CL Daley
In vitro synergy between clofazimine and amikacin in treatment of nontuberculous mycobacterial disease
Antimicrob Agents Chemother, 56 (2012), pp. 6324-632712
JR Dalovisio, GA Pankey, RJ Wallace, DB Jones
Clinical usefulness of amikacin and doxycycline in the treatment of infection due to Mycobacterium fortuitum and Mycobacterium chelonei
Rev Infect Dis, 3 (1981), pp. 1068-107413
WW Yew, SY Kwan, PC Wong, J Lee
Ofloxacin and imipenem in the treatment of Mycobacterium fortuitum and Mycobacterium chelonae lung infections
Tubercle, 71 (1990), pp. 131-13314
J van Ingen, BE Ferro, W Hoefsloot, MJ Boeree, D van Soolingen
Drug treatment of pulmonary nontuberculous mycobacterial disease in HIV-negative patients: the evidence
Expert Rev Anti Infect Ther, 11 (2013), pp. 1065-107715
GH Shen, BD Wu, ST Hu, CF Lin, KM Wu, JH Chen
High efficacy of clofazimine and its synergistic effect with amikacin against rapidly growing mycobacteria
Int J Antimicrob Agents, 35 (2010), pp. 400-40416
BA Brown-Elliott, KA Nash, RJ Wallace Jr
Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria
Clin Microbiol Rev, 25 (2012), pp. 545-58217
KA Nash, BA Brown-Elliott, RJ Wallace Jr
A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae
Antimicrob Agents Chemother, 53 (2009), pp. 1367-137618
RJ Wallace Jr, A Meier, BA Brown, et al.
Genetic basis for clarithromycin resistance among isolates of Mycobacterium chelonae and Mycobacterium abscessus
Antimicrob Agents Chemother, 40 (1996), pp. 1676-168119
IA George, CA Santos, MA Olsen, TC Bailey
Epidemiology and outcomes of nontuberculous mycobacterial infections in solid organ transplant recipients at a midwestern center
Transplantation, 100 (2016), pp. 1073-107820
T Goto, R Hamaguchi, A Maeshima, Y Oyamada, R Kato
Pulmonary resection for Mycobacterium chelonae infection
Ann Thorac Cardiovasc Surg, 18 (2012), pp. 128-13121
BM Knoll, S Kappagoda, RR Gill, et al.
Non-tuberculous mycobacterial infection among lung transplant recipients: a 15-year cohort study
Transpl Infect Dis, 14 (2012), pp. 452-46022
Y Ko, W Kim, BS Shin, et al.
Nontuberculous mycobacterial lung disease caused by Mycobacterium chelonae: A Case Report
Tuberc Respir Dis (Seoul), 74 (2013), pp. 191-19423
E Peres, Y Khaled, OI Krijanovski, et al.
Mycobacterium chelonae necrotizing pneumonia after allogeneic hematopoietic stem cell transplant: report of clinical response to treatment with tigecycline
Transpl Infect Dis, 11 (2009), pp. 57-6324
G Satyanarayana, SK Heysell, KW Scully, ER Houpt
Mycobacterial infections in a large Virginia hospital, 2001-2009
BMC Infect Dis, 11 (2011), p. 11325
M Tsukamura, E Nakamura, I Kurita, T Nakamura
Isolation of Mycobacterium chelonei subspecies chelonei (Mycobacterium borstelense) from pulmonary lesions of 9 patients
Am Rev Respir Dis, 108 (1973), pp. 683-68526
N Al Jarad, P Demertzis, DJ Jones, et al.
Comparison of characteristics of patients and treatment outcome for pulmonary non-tuberculous mycobacterial infection and pulmonary tuberculosis
Thorax, 51 (1996), pp. 137-13927
N Gupta, A Mittal, VKM Niyas, et al.
Nontuberculous mycobacteria: a report of eighteen cases from a tertiary care center in India
Lung India, 37 (2020), pp. 495-50028
D Hadjiliadis, A Adlakha, UB Prakash
Rapidly growing mycobacterial lung infection in association with esophageal disorders
Mayo Clin Proc, 74 (1999), pp. 45-5129
CW Huang, JH Chen, ST Hu, et al.
Synergistic activities of tigecycline with clarithromycin or amikacin against rapidly growing mycobacteria in Taiwan
Int J Antimicrob Agents, 41 (2013), pp. 218-22330
AD McCallum, SW Watkin, JF Faccenda
Non-tuberculous mycobacterial infections in the Scottish borders: identification, management and treatment outcomes--a retrospective review
J R Coll Physicians Edinb, 41 (2011), pp. 294-30331
RR Ruas, I Abreu, J Nuak, et al.
Nontuberculous mycobacteria in a tertiary hospital in Portugal: A clinical review
Int J Mycobacteriol, 6 (2017), pp. 344-34832
SK Shah, KJ McAnally, L Seoane, et al.
Analysis of pulmonary non-tuberculous mycobacterial infections after lung transplantation
Transpl Infect Dis, 18 (2016), pp. 585-59133
RJ Wallace Jr, G Dukart, BA Brown-Elliott, DE Griffith, EG Scerpella, B Marshall
Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections
J Antimicrob Chemother, 69 (2014), pp. 1945-195334
Y Yano, S Kitada, M Mori, et al.
Pulmonary disease caused by rapidly growing mycobacteria: a retrospective study of 44 cases in Japan
Respiration, 85 (2013), pp. 305-31135
S Park, GY Suh, MP Chung, et al.
Clinical significance of Mycobacterium fortuitum isolated from respiratory specimens
Respir Med, 102 (2008), pp. 437-44236
KA Nash, Y Zhang, BA Brown-Elliott, RJ Wallace Jr
Molecular basis of intrinsic macrolide resistance in clinical isolates of Mycobacterium fortuitum
J Antimicrob Chemother, 55 (2005), pp. 170-17737
RJ Wallace Jr, G Bedsole, G Sumter, et al.
Activities of ciprofloxacin and ofloxacin against rapidly growing mycobacteria with demonstration of acquired resistance following single-drug therapy
Antimicrob Agents Chemother, 34 (1990), pp. 65-7038
Y Abe, M Nakamura, K Suzuki, et al.
Massive hemoptysis due to Mycobacterium fortuitum infection controlled with bronchial artery embolization - a case report
Clin Imaging, 23 (1999), pp. 361-36339
H Al-Ghafli, S Al-Hajoj
Nontuberculous mycobacteria in Saudi Arabia and Gulf Countries: a review
Can Respir J, 2017 (2017), Article 503593240
JM Aronchick, WT Miller, DM Epstein, WB Gefter
Association of achalasia and pulmonary Mycobacterium fortuitum infection
Radiology, 160 (1986), pp. 85-8641
R Banerjee, R Hall, GR Hughes
Pulmonary Mycobacterium fortuitum infection in association with achalasia of the oesophagus. Case report and review of the literature
Br J Dis Chest, 64 (1970), pp. 112-11842
R Bouchentouf
Mycobacterium fortuitum infection associated with achalasia
Tunis Med, 96 (2018), pp. 311-31343
DN Burns, PK Rohatgi, R Rosenthal, M Seiler, FM Gordin
Disseminated Mycobacterium fortuitum successfully treated with combination therapy including ciprofloxacin
Am Rev Respir Dis, 142 (1990), pp. 468-47044
CY Chen, WH Sheng, CC Lai, et al.
Mycobacterial infections in adult patients with hematological malignancy
Eur J Clin Microbiol Infect Dis, 31 (2012), pp. 1059-106645
RF Corpe, CE Smith, I Stergus
Death due to Mycobacterium fortuitum
JAMA, 177 (1961), pp. 262-26346
KG de Mello, FC Mello, L Borga, et al.
Clinical and therapeutic features of pulmonary nontuberculous mycobacterial disease, Brazil, 1993-2011
Emerg Infect Dis, 19 (2013), pp. 393-39947
G Del Giudice, C Iadevaia, G Santoro, E Moscariello, R Smeraglia, C Marzo
Nontuberculous mycobacterial lung disease in patients without HIV infection: a retrospective analysis over 3 years
Clin Respir J, 5 (2011), pp. 203-21048
R Diel, F Ringshausen, E Richter, L Welker, J Schmitz, A Nienhaus
Microbiological and clinical outcomes of treating non-Mycobacterium avium complex nontuberculous mycobacterial pulmonary disease: a systematic review and meta-analysis
Chest, 152 (2017), pp. 120-14249
CM Ellender, DB Law, RM Thomson, GW Eather
Safety of IV amikacin in the treatment of pulmonary non-tuberculous mycobacterial disease
Respirology, 21 (2016), pp. 357-36250
C Garcia-Ibarbia, B Espina, M Fernandez-Ayala, D Nan
Mycobacterium fortuitum infection and lipoid pneumonia
Respiratory Medicine Extra, 3 (2007), pp. 92-9351
XY Han, I Dé, KL Jacobson
Rapidly growing mycobacteria: clinical and microbiologic studies of 115 cases
Am J Clin Pathol, 128 (2007), pp. 612-62152
A Hasan, TLN Swamy
Nocardia and Mycobacterium fortuitum infection in a case of lipoid pneumonia
Respir Med CME, 4 (2011), pp. 75-7853
RS Howard 2nd, JH Woodring, HM Vandiviere, ML Dillon
Mycobacterium fortuitum pulmonary infection complicating achalasia
South Med J, 84 (1991), pp. 1391-139354
S Ichiyama, M Tsukamura
Ofloxacin and the treatment of pulmonary disease due to Mycobacterium fortuitum
Chest, 92 (1987), pp. 1110-111255
MR Keating, JS Daly
Nontuberculous mycobacterial infections in solid organ transplantation
Am J Transplant, 13 (suppl 4) (2013), pp. 77-8256
E Kupeli, E Bozkurt, O Azap, FO Eyuboglu
Mycobacterium fortuitum infection presenting as community-acquired pneumonia in an immunocompetent host
J Bronchology Interv Pulmonol, 17 (2010), pp. 356-35857
K Kurokawa, N Harada, H Sasano, et al.
Pulmonary infection due to fluoroquinolone-resistant Mycolicibacterium fortuitum: a case report
BMC Infect Dis, 20 (2020), p. 86658
MP Lessing, MM Walker
Fatal pulmonary infection due to Mycobacterium fortuitum
J Clin Pathol, 46 (1993), pp. 271-27259
BA Lopez-Luis, J Sifuentes-Osornio, MT Pérez-Gutiérrez, B Chávez-Mazari, M Bobadilla-Del-Valle, A Ponce-de-León
Nontuberculous mycobacterial infection in a tertiary care center in Mexico, 2001-2017
Braz J Infect Dis, 24 (2020), pp. 213-22060
SL Martiniano, BD Wagner, A Levin, JA Nick, SD Sagel, CL Daley
Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial infection
Chest, 152 (2017), pp. 800-80961
T Matsumoto, K Otsuka, K Tomii
Mycobacterium fortuitum thoracic empyema: a case report and review of the literature
J Infect Chemother, 21 (2015), pp. 747-75062
C Napolitano, X Arunabh, A Mojaverian, R Shah, MR Kaplan
Mycobacterium fortuitum pulmonary infection complicating achalasia
Infect Dis Clin Pract, 12 (2004), pp. 178-18063
S Okamori, T Asakura, T Nishimura, et al.
Natural history of Mycobacterium fortuitum pulmonary infection presenting with migratory infiltrates: a case report with microbiological analysis
BMC Infect Dis, 18 (2018), p. 164
M Panagiotou, AI Papaioannou, K Kostikas, et al.
The epidemiology of pulmonary nontuberculous mycobacteria: data from a general hospital in Athens, Greece, 2007-2013
Pulm Med, 2014 (2014), Article 89497665
MR Radzniwan, H Tohid, S Ahmad, AF Mohd, F Md Anshar
Isolation of Mycobacterium fortuitum in sputum specimens of a patient with chronic cough: Is it clinically significant?
Malays Fam Physician, 9 (2014), pp. 38-4166
S Simons, J van Ingen, PR Hsueh, et al.
Nontuberculous mycobacteria in respiratory tract infections, eastern Asia
Emerg Infect Dis, 17 (2011), pp. 343-34967
J Vadakekalam, MJ Ward
Mycobacterium fortuitum lung abscess treated with ciprofloxacin
Thorax, 46 (1991), pp. 737-73868
G Varghese, R Shepherd, P Watt, JH Bruce
Fatal infection with Mycobacterium fortuitum associated with oesophageal achalasia
Thorax, 43 (1988), pp. 151-15269
CC Wang, MC Lin, JW Liu, YH Wang
Nontuberculous mycobacterial lung disease in southern Taiwan
Chang Gung Med J, 32 (2009), pp. 499-50870
JA Yu, MJ Weyant, JD Mitchell
Surgical treatment of atypical mycobacterial infections
Thorac Surg Clin, 22 (2012), pp. 277-28571
SH Annobil, GA Jamjoom, R Bobo, J Iyengar
Fatal lipoid pneumonia in an infant complicated by Mycobacterium fortuitum infection
Trop Geogr Med, 44 (1992), pp. 160-16472
SY Kim, SM Moon, BW Jhun, et al.
Species distribution and macrolide susceptibility of Mycobacterium fortuitum complex clinical isolates
Antimicrob Agents Chemother, 63 (2019), pp. e02331-e0241873
B Rammaert, LJ Couderc, E Rivaud, et al.
Mycobacterium genavense as a cause of subacute pneumonia in patients with severe cellular immunodeficiency
BMC Infect Dis, 11 (2011), p. 31174
M Mahmood, S Ajmal, OM Abu Saleh, A Bryson, JR Marcelin, JW Wilson
Mycobacterium genavense infections in non-HIV immunocompromised hosts: a systematic review
Infect Dis (Lond), 50 (2018), pp. 329-33975
W Hoefsloot, J van Ingen, EJ Peters, et al.
Mycobacterium genavense in the Netherlands: an opportunistic pathogen in HIV and non-HIV immunocompromised patients. An observational study in 14 cases
Clin Microbiol Infect, 19 (2013), pp. 432-43776
EC Böttger, A Teske, P Kirschner, et al.
Disseminated “Mycobacterium genavense” infection in patients with AIDS
Lancet, 340 (1992), pp. 76-8077
JJ Blanco Pérez, A Pérez González, LE Morano Amado, et al.
Clinical significance of environmental mycobacteria isolated from respiratory specimens of patients with and without silicosis
Arch Bronconeumol, 52 (2016), pp. 145-15078
C Bogdan, P Kern, E Richter, et al.
Systemic infection with Mycobacterium genavense following immunosuppressive therapy in a patient who was seronegative for human immunodeficiency virus
Clin Infect Dis, 24 (1997), pp. 1245-124779
JS Doggett, L Strasfeld
Disseminated Mycobacterium genavense with pulmonary nodules in a kidney transplant recipient: case report and review of the literature
Transpl Infect Dis, 13 (2011), pp. 38-4380
P Charles, O Lortholary, A Dechartres, et al.
Mycobacterium genavense infections: a retrospective multicenter study in France, 1996-2007
Medicine (Baltimore), 90 (2011), pp. 223-23081
EC Böttger
Mycobacterium genavense: an emerging pathogen
Eur J Clin Microbiol Infect Dis, 13 (1994), pp. 932-93682
CD Gaynor, RA Clark, FP Koontz, S Emler, B Hirschel, LS Schlesinger
Disseminated Mycobacterium genavense infection in two patients with AIDS
Clin Infect Dis, 18 (1994), pp. 455-45783
VO Thomsen, UB Dragsted, J Bauer, K Fuursted, J Lundgren
Disseminated infection with Mycobacterium genavense: a challenge to physicians and mycobacteriologists
J Clin Microbiol, 37 (1999), pp. 3901-390584
H Albrecht, S Rüsch-Gerdes, HJ Stellbrink, H Greten, S Jäckle
Disseminated Mycobacterium genavense infection as a cause of pseudo-Whipple's disease and sclerosing cholangitis
Clin Infect Dis, 25 (1997), pp. 742-74385
M Szturmowicz, I Siemion-Szcześniak, D Wyrostkiewicz, et al.
Factors predisposing to non-tuberculous mycobacterial lung disease in the patients with respiratory isolates of non-tuberculous mycobacteria
Adv Respir Med, 86 (2018), pp. 261-26786
W Hoefsloot, J van Ingen, C Andrejak, et al.
The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study
Eur Respir J, 42 (2013), pp. 1604-161387
JE Moore, ME Kruijshaar, LP Ormerod, F Drobniewski, I Abubakar
Increasing reports of non-tuberculous mycobacteria in England, Wales and Northern Ireland, 1995-2006
BMC Public Health, 10 (2010), p. 61288
MB Waak, TM LaPara, C Hallé, RM Hozalski
Nontuberculous mycobacteria in two drinking water distribution systems and the role of residual disinfection
Environ Sci Technol, 53 (2019), pp. 8563-857389
AS Oriani, MJ Marfil, MJ Zumárraga, MD Baldini
Prevalence and species diversity of nontuberculous mycobacteria in drinking water supply system of Bahía Blanca City, Argentina
Int J Mycobacteriol, 8 (2019), pp. 138-14590
A Bauernfeind
Comparative in-vitro activities of the new quinolone, Bay y 3118, and ciprofloxacin, sparfloxacin, tosufloxacin, CI-960 and CI-990
J Antimicrob Chemother, 31 (1993), pp. 505-52291
B Goswami, P Narang, PS Mishra, R Narang, U Narang, DK Mendiratta
Drug susceptibility of rapid and slow growing non-tuberculous mycobacteria isolated from symptomatics for pulmonary tuberculosis, Central India
Indian J Med Microbiol, 34 (2016), pp. 442-44792
BA Brown-Elliott, CJ Crist, LB Mann, RW Wilson, RJ Wallace Jr
In vitro activity of linezolid against slowly growing nontuberculous mycobacteria
Antimicrob Agents Chemother, 47 (2003), pp. 1736-173893
CP Craig, SM Kreitzer
Non-tuberculous mycobacterial infections: human infections due to Mycobacterium gordonae
Infect Dis Rep, 6 (1980), pp. 79-8894
HY Chang, WC Tsai, TF Lee, WH Sheng
Mycobacterium gordonae infection in immunocompromised and immunocompetent hosts: a series of seven cases and literature review
J Formos Med Assoc, 120 (2021), pp. 524-53295
J de Gracia, R Vidal, N Martin, C Bravo, T Gonzalez, A Riba
Pulmonary disease caused by Mycobacterium gordonae
Tubercle, 70 (1989), pp. 135-13796
JG Douglas, MA Calder, YF Choo-Kang, AG Leitch
Mycobacterium gordonae: a new pathogen?
Thorax, 41 (1986), pp. 152-15397
K Morimoto, Y Kazumi, Y Shiraishi, et al.
Clinical and microbiological features of definite Mycobacterium gordonae pulmonary disease: the establishment of diagnostic criteria for low-virulence mycobacteria
Trans R Soc Trop Med Hyg, 109 (2015), pp. 589-59398
U Nanda Kumar, B Varkey
Pulmonary infection caused by Mycobacterium gordonae
Br J Dis Chest, 74 (1980), pp. 189-19299
V Nathan, JB Mehta, W Dralle
Rifabutin in the treatment of cavitary lung disease due to Mycobacterium gordonae
South Med J, 86 (1993), pp. 839-841100
Y Umeda, Y Matsuno, M Imaizumi, Y Mori, H Iwata, H Takiya
Extralobar pulmonary sequestration infected with Mycobacterium gordonae
J Thorac Cardiovasc Surg, 137 (2009), pp. e23-e24101
KH Schroder, I Juhlin
Mycobacterium malmoense sp.nov
Int J Syst Bacteriol, 26 (1977), p. 409102
W Hoefsloot, J van Ingen, WC de Lange, PN Dekhuijzen, MJ Boeree, D van Soolingen
Clinical relevance of Mycobacterium malmoense isolation in the Netherlands
Eur Respir J, 34 (2009), pp. 926-931103
MT Henry, L Inamdar, D O'Riordain, M Schweiger, JP Watson
Nontuberculous mycobacteria in non-HIV patients: epidemiology, treatment and response
Eur Respir J, 23 (2004), pp. 741-746104
Research Committee of the British Thoracic Society
First randomised trial of treatments for pulmonary disease caused by M avium intracellulare, M malmoense, and M xenopi in HIV negative patients: rifampicin, ethambutol and isoniazid versus rifampicin and ethambutol
Thorax, 56 (2001), pp. 167-172105
British Thoracic Society
Pulmonary disease caused by M. malmoense in HIV negative patients: 5-yr follow-up of patients receiving standardised treatment
Eur Respir J, 21 (2003), pp. 478-482106
J van Ingen, T van der Laan, R Dekhuijzen, M Boeree, D van Soolingen
In vitro drug susceptibility of 2275 clinical non-tuberculous mycobacterium isolates of 49 species in the Netherlands
Int J Antimicrob Agents, 35 (2010), pp. 169-173107
P Engervall, M Björkholm, B Petrini, N Heurlin, B Henriques, G Källenius
Disseminated Mycobacterium malmoense infection in a patient with chronic granulocytic leukaemia
J Intern Med, 234 (1993), pp. 231-233108
H Saito, H Tomioka, K Sato, H Tasaka, S Dekio
Mycobacterium malmoense isolated from soil
Microbiol Immunol, 38 (1994), pp. 313-315109
MF Thorel, JO Falkinham 3rd, RG Moreau
Environmental mycobacteria from alpine and subalpine habitats
FEMS Microbiol Ecol, 49 (2004), pp. 343-347110
AJ Evans, AJ Crisp, A Colville, SA Evans, ID Johnston
Pulmonary infections caused by Mycobacterium malmoense and Mycobacterium tuberculosis: comparison of radiographic features
AJR Am J Roentgenol, 161 (1993), pp. 733-737111
AJ France, DT McLeod, MA Calder, A Seaton
Mycobacterium malmoense infections in Scotland: an increasing problem
Thorax, 42 (1987), pp. 593-595112
W Hoefsloot, MJ Boeree, J van Ingen, et al.
The rising incidence and clinical relevance of Mycobacterium malmoense: a review of the literature
Int J Tuberc Lung Dis, 12 (2008), pp. 987-993113
J Banks, PA Jenkins, AP Smith
Pulmonary infection with Mycobacterium malmoense--a review of treatment and response
Tubercle, 66 (1985), pp. 197-203114
MP Murray, IF Laurenson, AT Hill
Outcomes of a standardized triple-drug regimen for the treatment of nontuberculous mycobacterial pulmonary infection
Clin Infect Dis, 47 (2008), pp. 222-224115
PA Jenkins, IA Campbell, J Banks, CM Gelder, RJ Prescott, AP Smith
Clarithromycin vs ciprofloxacin as adjuncts to rifampicin and ethambutol in treating opportunist mycobacterial lung diseases and an assessment of Mycobacterium vaccae immunotherapy
Thorax, 63 (2008), pp. 627-634116
J van Ingen, MJ Boeree, PN Dekhuijzen, D van Soolingen
Clinical relevance of Mycobacterium simiae in pulmonary samples
Eur Respir J, 31 (2008), pp. 106-109117
DL Rynkiewicz, GD Cage, WR Butler, NM Ampel
Clinical and microbiological assessment of Mycobacterium simiae isolates from a single laboratory in southern Arizona
Clin Infect Dis, 26 (1998), pp. 625-630118
G Valero, J Peters, JH Jorgensen, JR Graybill
Clinical isolates of Mycobacterium simiae in San Antonio, Texas. An 11-yr review
Am J Respir Crit Care Med, 152 (1995), pp. 1555-1557119
JF Jabbour, A Hamieh, SL Sharara, SS Kanj
Mycobacterium simiae: harmless colonizer or deadly pathogen?
PLoS Pathog, 16 (2020), Article e1008418120
E Braun, H Sprecher, S Davidson, I Kassis
Epidemiology and clinical significance of non-tuberculous mycobacteria isolated from pulmonary specimens
Int J Tuberc Lung Dis, 17 (2013), pp. 96-99121
D Shitrit, N Peled, J Bishara, et al.
Clinical and radiological features of Mycobacterium kansasii infection and Mycobacterium simiae infection
Respir Med, 102 (2008), pp. 1598-1603122
A Hamieh, R Tayyar, H Tabaja, et al.
Emergence of Mycobacterium simiae: A retrospective study from a tertiary care center in Lebanon
PLoS One, 13 (2018), Article e0195390123
MJ Nasiri, H Dabiri, D Darban-Sarokhalil, A Hashemi Shahraki
prevalence of non-tuberculosis mycobacterial infections among tuberculosis suspects in Iran: systematic review and meta-analysis
PLoS One, 10 (2015), Article e0129073124
S Shenai, C Rodrigues, A Mehta
Time to identify and define non-tuberculous mycobacteria in a tuberculosis-endemic region
Int J Tuberc Lung Dis, 14 (2010), pp. 1001-1008125
J van Ingen, SE Totten, LB Heifets, MJ Boeree, CL Daley
Drug susceptibility testing and pharmacokinetics question current treatment regimens in Mycobacterium simiae complex disease
Int J Antimicrob Agents, 39 (2012), pp. 173-176126
H Pang, Y Jiang, K Wan
Drug susceptibility of 33 reference strains of slowly growing mycobacteria to 19 antimicrobial agents
BioMed Res Int, 2017 (2017), Article 1584658127
S Cowman, K Burns, S Benson, R Wilson, MR Loebinger
The antimicrobial susceptibility of non-tuberculous mycobacteria
J Infect, 72 (2016), pp. 324-331128
P Baghaei, P Tabarsi, P Farnia, et al.
Pulmonary disease caused by Mycobacterium simiae in Iran's national referral center for tuberculosis
J Infect Dev Ctries, 6 (2012), pp. 23-28129
N Coolen-Allou, T Touron, O Belmonte, et al.
Clinical, radiological, and microbiological characteristics of Mycobacterium simiae infection in 97 patients
Antimicrob Agents Chemother, 62 (2018), pp. e00395-e00418130
A Hashemi-Shahraki, D Darban-Sarokhalil, P Heidarieh, et al.
Mycobacterium simiae: a possible emerging pathogen in Iran
Jpn J Infect Dis, 66 (2013), pp. 475-479131
SH Jeong, SY Kim, H Lee, et al.
Nontuberculous mycobacterial lung disease caused by Mycobacterium simiae: the first reported case in South Korea
Tuberc Respir Dis (Seoul), 78 (2015), pp. 432-435132
A Aharon, P Langevitz, R Maran, D Blank-Porat, S Shtrasburg, A Livneh
Reactive amyloidosis in a patient with Mycobacterium simiae pulmonary infection
Respir Med, 92 (1998), pp. 123-124133
HD Rose, GJ Dorff, M Lauwasser, NK Sheth
Pulmonary and disseminated Mycobacterium simiae infection in humans
Am Rev Respir Dis, 126 (1982), pp. 1110-1113134
LM Reddy, H Bhaskar, N Prashanth, NK Kumar Narahari, GK Paramjyothi
All Mycobacteria are not tubercular: A case report
J Clin Diagn Res, 12 (2018), pp. OD01-OD03135
FG McSweeney, ME O'Brien, S Sheehan, B Plant, G Corcoran
Pulmonary Mycobacterium szulgai infection
Ir Med J, 105 (2012), pp. 275-277136
J van Ingen, MJ Boeree, WC de Lange, PE de Haas, PN Dekhuijzen, D van Soolingen
Clinical relevance of Mycobacterium szulgai in the Netherlands
Clin Infect Dis, 46 (2008), pp. 1200-1205137
JM Maloney, CR Gregg, DS Stephens, FA Manian, D Rimland
Infections caused by Mycobacterium szulgai in humans
Rev Infect Dis, 9 (1987), pp. 1120-1126138
WB Schaefer, E Wolinsky, PA Jenkins, J Marks
Mycobacterium szulgai-a new pathogen. Serologic identification and report of five new cases
Am Rev Respir Dis, 108 (1973), pp. 1320-1326139
PT Davidson
Mycobacterium szulgai; a new pathogen causing infection of the lung
Chest, 69 (1976), pp. 799-801140
AE Medinger, SV Spagnolo
Mycobacterium szulgai pulmonary infection: the importance of knowing
South Med J, 74 (1981), pp. 85-86141
J Collazos, F Díaz, J Rodriguez, R Ayarza
Persistent lung infection due to Mycobacterium szulgai
Tuber Lung Dis, 74 (1993), pp. 412-413142
DA Benator, V Kan, FM Gordin
Mycobacterium szulgai infection of the lung: case report and review of an unusual pathogen
Am J Med Sci, 313 (1997), pp. 346-351143
E Tortoli, G Besozzi, C Lacchini, V Penati, MT Simonetti, S Emler
Pulmonary infection due to Mycobacterium szulgai, case report and review of the literature
Eur Respir J, 11 (1998), pp. 975-977144
K Tsuyuguchi, R Amitani, H Matsumoto, et al.
A resected case of Mycobacterium szulgai pulmonary disease
Int J Tuberc Lung Dis, 2 (1998), pp. 258-260145
S Nakayama, T Fujii, J Kadota, et al.
Pulmonary mycobacteriosis caused by rifampicin-resistant Mycobacterium szulgai
Intern Med, 39 (2000), pp. 309-312146
Y Tsunezuka, H Sato, C Hiranuma
Surgical outcome of mycobacterium other than Mycobacterium tuberculosis pulmonary disease
Thorac Cardiovasc Surg, 48 (2000), pp. 290-293147
JM Sánchez-Alarcos, J De Miguel-Díez, I Bonilla, JJ Sicilia, JL Alvarez-Sala
Pulmonary infection due to Mycobacterium szulgai
Respiration, 70 (2003), pp. 533-536148
M Hotta, Y Minami, I Itoda, K Yoshimori, K Takano
A young female patient with anorexia nervosa complicated by Mycobacterium szulgai pulmonary infection
Int J Eat Disord, 35 (2004), pp. 115-119149
M Gutierrez, M Feola, L Lenge, R Rey, M Hoffman
First pulmonary case reported in Argentina of infection with mycobacterium szulgai, a rare pathogen
J Clin Microbiol, 45 (2007), pp. 3121-3124







暂无评论内容